Carbon biomass mesozooplankton estimates from MOCNESS oblique tows from R/V Thomas G. Thompson TT043, TT045, TT050 cruises in the Arabian Sea in 1995 (U.S. JGOFS Arabian Sea project)
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Roman, Michael R. | University of Maryland Center for Environmental Science (UMCES/HPL) | Principal Investigator |
| Gauzens, Anne | University of Maryland Center for Environmental Science (UMCES/HPL) | Co-Principal Investigator |
| Chandler, Cynthia L. | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Carbon biomass mesozooplankton, MOCNESS oblique tows
See Platform deployments for cruise specific documentation
Analysis methodology referenced in:
Roman, M.R. et al, Zooplankton variability on the equator at 140W during the JGOFS EqPac Study. Deep Sea Research II, 42(2-3) : 673-693, 1995
Mesozooplankton Grazing - Isotope Method
Measurements of grazing rates by the radioisotope uptake technique were done day and night, immediately following the MOCNESS tows, in short term, in situ incubations at 1, 10, 30, 50, 70 and 90 m (Roman and Rublee, 1981). Five-liter Plexiglas chambers (General Oceanics) with 64 µm-mesh covering the bottom were lowered 10 m past the desired depths and then raised to concentrate zooplankton in the chambers. A messenger triggered the close of the bottles and released the radioisotope tracers (25 µCi l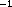 of methyl
of methyl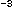 H-thymidine, > 75 Ci mmol
H-thymidine, > 75 Ci mmol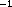 and 50 µCi l
and 50 µCi l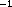 Na
Na
 CO
CO , 55.0 Ci mmol
, 55.0 Ci mmol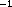 ) into the chambers. After a 45-minute incubation on the hydrowire, the chambers were retrieved, zooplankton collected on nested 200 and 64 µm sieves. The zooplankton were rinsed (filtered seawater, 10% HCl and deionized water) onto preweighed 12 µm pore-size Nuclepore filters and dried. Using a dissecting microscope, visible detritus and phytoplankton were removed with a sable brush. The filters were weighed and the weight-specific dpm of the isotopes were measured. Zooplankton carbon was assumed to be 40% of their dry weight to calculate the dpm mg C
) into the chambers. After a 45-minute incubation on the hydrowire, the chambers were retrieved, zooplankton collected on nested 200 and 64 µm sieves. The zooplankton were rinsed (filtered seawater, 10% HCl and deionized water) onto preweighed 12 µm pore-size Nuclepore filters and dried. Using a dissecting microscope, visible detritus and phytoplankton were removed with a sable brush. The filters were weighed and the weight-specific dpm of the isotopes were measured. Zooplankton carbon was assumed to be 40% of their dry weight to calculate the dpm mg C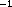 of the zooplankton. The labelled particulate matter (< 64 µm) was collected on 0.2 µm and 2.0 µm pore-size Nuclepore filters to determine the specific activity of the particulate matter. We derived weight-specific corrections for the absorption and adsorption of the isotopes in shipboard experiments using both filtered seawater and time-0 controls. These corrections were generally less than 10% of experimental values. The isotope activity of the labelled particulate matter (> 2 µm) and zooplankton were used to calculate zooplankton filtration rates, F = liters filtered. mg zooplankton C
of the zooplankton. The labelled particulate matter (< 64 µm) was collected on 0.2 µm and 2.0 µm pore-size Nuclepore filters to determine the specific activity of the particulate matter. We derived weight-specific corrections for the absorption and adsorption of the isotopes in shipboard experiments using both filtered seawater and time-0 controls. These corrections were generally less than 10% of experimental values. The isotope activity of the labelled particulate matter (> 2 µm) and zooplankton were used to calculate zooplankton filtration rates, F = liters filtered. mg zooplankton C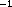 h
h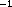 , after Daro (1978). The grazing impact of the zooplankton community, expressed as liters filtered m
, after Daro (1978). The grazing impact of the zooplankton community, expressed as liters filtered m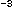 h
h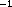 , was calculated as the product of the weight-specific filtration rate determined from the in situ incubations and the zooplankton biomass in the same depth interval determined immediately prior to the grazing incubation. We used both
, was calculated as the product of the weight-specific filtration rate determined from the in situ incubations and the zooplankton biomass in the same depth interval determined immediately prior to the grazing incubation. We used both  C bicarbonate and [methyl]
C bicarbonate and [methyl] 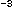 H thymidine to estimate the filtration rates of mesozooplankton upon autotrophs (phytoplankton and protozoa that consumed phytoplankton) and heterotrophs (free-living and attached bacteria and protozoa that have consumed bacteria).
H thymidine to estimate the filtration rates of mesozooplankton upon autotrophs (phytoplankton and protozoa that consumed phytoplankton) and heterotrophs (free-living and attached bacteria and protozoa that have consumed bacteria).
Mesozooplankton Grazing - Gut Fluorescence Method
Zooplankton were collected day and night, immediately preceding MOCNESS tows, from vertical tows with opening-closing, 3/4 meter-Puget Sound nets equipped with 200 µm mesh and solid cod ends. Nets were towed to sample depth strata similar to those sampled by the MOCNESS in the upper 120 m. Three depth strata sampled were: 0-20 m, 20-60 m and 60-120 m. The contents of each net was immediately sieved under dim light red light to yield three size fractions: 2.0-1.0 mm; 1.0-0.5 mm and 0.5-0.2 mm. Each fraction was immediately filtered onto a glass fiber filter (GF/A) and frozen at -20 C. This procedure took about 5 min. Samples were analyzed in the laboratory ashore. We employed a modification of the procedure described by Dam and Peterson (1988). Thawed samples were placed under a dissecting scope, animals picked individually with jeweler's forceps without regard to species, gently rinsed with 0.2 µm-filtered seawater and placed in centrifuge tubes containing 90% high-grade, cold acetone solution. The whole procedure was performed under dim, red-light illumination. We typically took triplicate subsamples of 30-100 animals, with the number of individuals depending on the size fraction considered. A similar number of animals per sample was also picked for weight (C,N) determination. The tubes containing the acetone and animals were kept frozen at -20
C. This procedure took about 5 min. Samples were analyzed in the laboratory ashore. We employed a modification of the procedure described by Dam and Peterson (1988). Thawed samples were placed under a dissecting scope, animals picked individually with jeweler's forceps without regard to species, gently rinsed with 0.2 µm-filtered seawater and placed in centrifuge tubes containing 90% high-grade, cold acetone solution. The whole procedure was performed under dim, red-light illumination. We typically took triplicate subsamples of 30-100 animals, with the number of individuals depending on the size fraction considered. A similar number of animals per sample was also picked for weight (C,N) determination. The tubes containing the acetone and animals were kept frozen at -20  C for at least 24 h for pigment extraction. The amount of pigment (chlorophyll and phaeopigments) per individual was measured fluorometrically and expressed in terms of chlorophyll (body carbon)
C for at least 24 h for pigment extraction. The amount of pigment (chlorophyll and phaeopigments) per individual was measured fluorometrically and expressed in terms of chlorophyll (body carbon)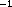 (Dam et al., 1993).
(Dam et al., 1993).
To convert gut pigment values to pigment ingestion rates, knowledge of the gut passage time is necessary. This is typically estimated from the reciprocal of the gut clearance rate constant (GCRC, units of time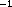 ) of animals placed in filtered seawater (review in Dam and Peterson, 1988). GCRC estimated over the first 30-40 min of gut evacuation is not statistically different from the gut evacuation rate constant of feeding animals (Kiorboe and Tiselius, 1987; Ellis and Small, 1990). We estimated GCRC every time collections for gut pigment were made. To collect animals for gut evacuation experiments, one tow (upper 60 m) was done immediately after the ones for gut fluorescence. We estimated GCRC of animals in the different size fractions (0.2-0.5 mm; 0.5-1.0 mm; 1.0-2-0 mm) by employing the procedure described by Small et al. (1989); i.e., animals were quickly sorted into separate containers, kept in the dark in a temperature-controlled room. We monitored the decline in gut pigment, at 5-min. intervals, over the first 25 min of gut evacuation. Seventy-five per cent of the variance of GCRC is explained by temperature, with a Q
) of animals placed in filtered seawater (review in Dam and Peterson, 1988). GCRC estimated over the first 30-40 min of gut evacuation is not statistically different from the gut evacuation rate constant of feeding animals (Kiorboe and Tiselius, 1987; Ellis and Small, 1990). We estimated GCRC every time collections for gut pigment were made. To collect animals for gut evacuation experiments, one tow (upper 60 m) was done immediately after the ones for gut fluorescence. We estimated GCRC of animals in the different size fractions (0.2-0.5 mm; 0.5-1.0 mm; 1.0-2-0 mm) by employing the procedure described by Small et al. (1989); i.e., animals were quickly sorted into separate containers, kept in the dark in a temperature-controlled room. We monitored the decline in gut pigment, at 5-min. intervals, over the first 25 min of gut evacuation. Seventy-five per cent of the variance of GCRC is explained by temperature, with a Q
 of 2.2 (Dam and Peterson, 1988). Therefore, we corrected GCRC for differences in temperature from the depth at which animals were collected. Weight-specific ingestion rates for each depth layer were estimated from knowledge of gut pigment and GCRC. The grazing impact of zooplankton was estimated from the product of weight-specific ingestion rates and biomass for each size class.
of 2.2 (Dam and Peterson, 1988). Therefore, we corrected GCRC for differences in temperature from the depth at which animals were collected. Weight-specific ingestion rates for each depth layer were estimated from knowledge of gut pigment and GCRC. The grazing impact of zooplankton was estimated from the product of weight-specific ingestion rates and biomass for each size class.
Literature Cited
Dam, H.G., C.A. Miller and S.H. Jonasdottir (1993). The trophic role of mesozooplankton at 47 N, 20
N, 20 W during the North Atlantic Bloom Experiment. Deep-Sea Research, 40: 197-212.
W during the North Atlantic Bloom Experiment. Deep-Sea Research, 40: 197-212.
Dam, H.G. and W.T. Peterson (1988). The effect of temperature on the gut clearance rate constant of planktonic copepods. Journal of Experimental Marine Biology and Ecology, 123: 1-14.
Daro, M.H. (1978). A simplified  C method for grazing measurements on natural planktonic populations. Helgolander wiss. Meeresunters, 31: 241-248.
C method for grazing measurements on natural planktonic populations. Helgolander wiss. Meeresunters, 31: 241-248.
Ellis, S.G. and L.F. Small (1989). A comparison of gut evacuation rates of feeding and non-feeding Calanus marshallae. Marine Biology, 103: 175-181.
Kiorboe, T. and P. Tiselius (1987). Gut clearance and pigment destruction in a herbivorous copepod, Acartia tonsa, and the determination of in situ grazing rates. Journal of Plankton Research, 9: 525-534.
Roman, M.R. and P.A. Rublee (1981). A method to determine in situ grazing rates on natural particle assemblages. Marine Biology, 65: 303-309.
Small, L.F., M.R. Landry, R.W. Eppley, F. Azam and A.F. Carlucci (1989). Role of plankton in the carbon and nitrogen budgets of the Santa Monica Basin, California. Marine Ecology Progress Series, 56: 57-74.
Wiebe, P.H., A.W. Morton, A.M. Bradley, R.H. Backus, J.E. Craddock T.J. Cowles, V.A. Barber and G.R. Flierl (1985). New developments in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Marine Biology, 87: 313-323.
| File |
|---|
mesozoo_moc_TT043.csv (Comma Separated Values (.csv), 4.56 KB) MD5:5e7c59e3252ccccf090f6af58412c27b version April 23, 1996 Mesozooplankton carbon biomass (CHN analysis) Michael Roman, Anne Gauzens Process 1, Arabian Sea Cruise TN043 MOCNESS 1/4 Sampler - oblique tows from 200m to surface |
mesozoo_moc_TT045.csv (Comma Separated Values (.csv), 3.96 KB) MD5:300683562d7187472843c6e5b74f171b version April 23, 1996 Mesozooplankton carbon biomass (CHN analysis) Michael Roman, Anne Gauzens Process 2, Arabian Sea Cruise TN045 MOCNESS 1/4 Sampler - oblique tows from 200m to surface |
mesozoo_moc_TT050.csv (Comma Separated Values (.csv), 3.48 KB) MD5:2492c76e83e32769f4740845ad5eedbd version April 23, 1996 Mesozooplankton carbon biomass (CHN analysis) Michael Roman, Anne Gauzens Process 5, Arabian Sea Cruise TN050 - submitted by Michael Roman MOCNESS 1/4 Sampler - oblique tows from 200m to surface |
| Parameter | Description | Units |
| sta | station number | |
| sta_std | Arabian Sea standard station identifier | |
| event | event number, from event log | |
| depth_begin | depth sample collection started | meters |
| depth_end | depth sample collection stopped | meters |
| depth_mid | mid point of sample collection depth range | meters |
| mzp_C_64_200 | carbon biomass of mesozooplankton in size fraction 64-200 micrometers | mmol C m^2 |
| mzp_C_gt_200 | carbon biomass of mesozooplankton in size fraction greater than 200 micrometers | mmol C m^2 |
| Dataset-specific Instrument Name | MOCNESS.25 |
| Generic Instrument Name | MOCNESS.25 |
| Dataset-specific Description | Mesozooplankton (> 64 µm) were collected from day/night pairs of tows with a 0.25 m-mouth area MOCNESS equipped with nine nets with a 7:1, mouth:length ratio (Wiebe et al., 1985). |
| Generic Instrument Description | The Multiple Opening/Closing Net and Environmental Sensing System or MOCNESS is a family of net systems based on the Tucker Trawl principle. The MOCNESS-1/4 carries nine 1/4-m2 nets usually of 64 micrometer mesh and is used to sample the larger micro-zooplankton. |
TT043
| Website | |
| Platform | R/V Thomas G. Thompson |
| Report | |
| Start Date | 1995-01-08 |
| End Date | 1995-02-05 |
| Description | Purpose: Process Cruise #1 (Late NE Monsoon) Methods & Sampling PI: Michael Roman of: Horn Point Environmental Laboratory dataset: Carbon biomass mesozooplankton, MOCNESS oblique tows dates: January 12, 1995 to January 30, 1995 location: N: 19.1744 S: 9.9994 W: 58.0005 E: 68.7449 project/cruise: Process 1 TN043 Winter monsoon in the Arabian Sea ship: R/V Thomas Thompson Mesozooplankton Protocols Hans Dam and Michael Roman Mesozooplankton Biomass Mesozooplankton (> 64 µm) were collected from day/night pairs of tows with a 0.25 m-mouth area MOCNESS equipped with nine nets with a 7:1, mouth:length ratio (Wiebe et al., 1985). Transmission by underwater sensors on the MOCNESS through conducting cable to the deck yielded output of fluorescence, temperature, conductivity, depth, frame angle, volume filtered, and net closing response at 1-s intervals. Oblique tows were taken from 1000 m and 200 m to the surface. Eight depth strata were sampled on both shallow and deep tows: 0 - 10 m 0 - 20 m 10 - 20 m 20 - 40 m 20 - 40 m 40 - 100 m 40 - 60 m 100 - 200 m 60 - 80 m 200 - 400 m 80 - 100 m 400 - 600 m 100 - 150 m 600 - 800 m 150 - 200 m 800 - 1000 m Descent rates of the MOCNESS were generally between 40 and 50 m min and ascent speeds between 10 and 20 m min. The MOCNESS was towed at a ship speed of 2 kt which resulted in a net angle of near 45 . On board ship, the contents of each net were split in half with a Folsom Plankton Splitter. One half of the sample was preserved in 4% buffered (Sodium Borate) Formalin. The remaining half of the sample was gently wet-sieved through a 2.0 mm mesh to remove gelatinous zooplankton and micronekton (not caught quantitatively by 0.25 m MOCNESS). The portion passing through this mesh was wet-sieved further through 1.0 mm, 0.5 mm and 0.2 mm meshes. This procedure yielded four different size classes: 2.0-1.0 mm; 1.0-0.5 mm; 0.5-0.2 mm and 0.2-0.064 mm. The samples caught on these size fractions were diluted and thoroughly mixed in a known volume of filtered seawater and duplicate aliquots, drawn with a Hensen-Stempel pipette, filtered onto precombusted GF/D filters and rinsed with a small amount of distilled water to get rid of salt. Filters were dried at 60 C. Organic carbon and nitrogen for each filter were measured with a Model 440 Control Equipment CHN analyzer at the Horn Point Laboratory of the University of Maryland. The average error associated with subsampling the zooplankton catch for carbon analysis was 16% (standard deviation/mean). Zooplankton biomass of the different size fractions as well as the amount of total (> 64 µm) mesozooplankton is expressed as mg C m or mg C m(integrated). Processing Description Analysis methodology referenced in: Roman, M.R. et al, Zooplankton variability on the equator at 140W during the JGOFS EqPac Study. Deep Sea Research II, 42(2-3) : 673-693, 1995 Mesozooplankton Grazing - Isotope Method Measurements of grazing rates by the radioisotope uptake technique were done day and night, immediately following the MOCNESS tows, in short term, in situ incubations at 1, 10, 30, 50, 70 and 90 m (Roman and Rublee, 1981). Five-liter Plexiglas chambers (General Oceanics) with 64 µm-mesh covering the bottom were lowered 10 m past the desired depths and then raised to concentrate zooplankton in the chambers. A messenger triggered the close of the bottles and released the radioisotope tracers (25 µCi l of methylH-thymidine, > 75 Ci mmol and 50 µCi l Na CO, 55.0 Ci mmol ) into the chambers. After a 45-minute incubation on the hydrowire, the chambers were retrieved, zooplankton collected on nested 200 and 64 µm sieves. The zooplankton were rinsed (filtered seawater, 10% HCl and deionized water) onto preweighed 12 µm pore-size Nuclepore filters and dried. Using a dissecting microscope, visible detritus and phytoplankton were removed with a sable brush. The filters were weighed and the weight-specific dpm of the isotopes were measured. Zooplankton carbon was assumed to be 40% of their dry weight to calculate the dpm mg C of the zooplankton. The labelled particulate matter (< 64 µm) was collected on 0.2 µm and 2.0 µm pore-size Nuclepore filters to determine the specific activity of the particulate matter. We derived weight-specific corrections for the absorption and adsorption of the isotopes in shipboard experiments using both filtered seawater and time-0 controls. These corrections were generally less than 10% of experimental values. The isotope activity of the labelled particulate matter (> 2 µm) and zooplankton were used to calculate zooplankton filtration rates, F = liters filtered. mg zooplankton C h, after Daro (1978). The grazing impact of the zooplankton community, expressed as liters filtered m h, was calculated as the product of the weight-specific filtration rate determined from the in situ incubations and the zooplankton biomass in the same depth interval determined immediately prior to the grazing incubation. We used both C bicarbonate and [methyl] H thymidine to estimate the filtration rates of mesozooplankton upon autotrophs (phytoplankton and protozoa that consumed phytoplankton) and heterotrophs (free-living and attached bacteria and protozoa that have consumed bacteria). Mesozooplankton Grazing - Gut Fluorescence Method Zooplankton were collected day and night, immediately preceding MOCNESS tows, from vertical tows with opening-closing, 3/4 meter-Puget Sound nets equipped with 200 µm mesh and solid cod ends. Nets were towed to sample depth strata similar to those sampled by the MOCNESS in the upper 120 m. Three depth strata sampled were: 0-20 m, 20-60 m and 60-120 m. The contents of each net was immediately sieved under dim light red light to yield three size fractions: 2.0-1.0 mm; 1.0-0.5 mm and 0.5-0.2 mm. Each fraction was immediately filtered onto a glass fiber filter (GF/A) and frozen at -20 C. This procedure took about 5 min. Samples were analyzed in the laboratory ashore. We employed a modification of the procedure described by Dam and Peterson (1988). Thawed samples were placed under a dissecting scope, animals picked individually with jeweler's forceps without regard to species, gently rinsed with 0.2 µm-filtered seawater and placed in centrifuge tubes containing 90% high-grade, cold acetone solution. The whole procedure was performed under dim, red-light illumination. We typically took triplicate subsamples of 30-100 animals, with the number of individuals depending on the size fraction considered. A similar number of animals per sample was also picked for weight (C,N) determination. The tubes containing the acetone and animals were kept frozen at -20 C for at least 24 h for pigment extraction. The amount of pigment (chlorophyll and phaeopigments) per individual was measured fluorometrically and expressed in terms of chlorophyll (body carbon) (Dam et al., 1993). To convert gut pigment values to pigment ingestion rates, knowledge of the gut passage time is necessary. This is typically estimated from the reciprocal of the gut clearance rate constant (GCRC, units of time) of animals placed in filtered seawater (review in Dam and Peterson, 1988). GCRC estimated over the first 30-40 min of gut evacuation is not statistically different from the gut evacuation rate constant of feeding animals (Kiorboe and Tiselius, 1987; Ellis and Small, 1990). We estimated GCRC every time collections for gut pigment were made. To collect animals for gut evacuation experiments, one tow (upper 60 m) was done immediately after the ones for gut fluorescence. We estimated GCRC of animals in the different size fractions (0.2-0.5 mm; 0.5-1.0 mm; 1.0-2-0 mm) by employing the procedure described by Small et al. (1989); i.e., animals were quickly sorted into separate containers, kept in the dark in a temperature-controlled room. We monitored the decline in gut pigment, at 5-min. intervals, over the first 25 min of gut evacuation. Seventy-five per cent of the variance of GCRC is explained by temperature, with a Q of 2.2 (Dam and Peterson, 1988). Therefore, we corrected GCRC for differences in temperature from the depth at which animals were collected. Weight-specific ingestion rates for each depth layer were estimated from knowledge of gut pigment and GCRC. The grazing impact of zooplankton was estimated from the product of weight-specific ingestion rates and biomass for each size class. Literature Cited Dam, H.G., C.A. Miller and S.H. Jonasdottir (1993). The trophic role of mesozooplankton at 47 N, 20 W during the North Atlantic Bloom Experiment. Deep-Sea Research, 40: 197-212. Dam, H.G. and W.T. Peterson (1988). The effect of temperature on the gut clearance rate constant of planktonic copepods. Journal of Experimental Marine Biology and Ecology, 123: 1-14. Daro, M.H. (1978). A simplified C method for grazing measurements on natural planktonic populations. Helgolander wiss. Meeresunters, 31: 241-248. Ellis, S.G. and L.F. Small (1989). A comparison of gut evacuation rates of feeding and non-feeding Calanus marshallae. Marine Biology, 103: 175-181. Kiorboe, T. and P. Tiselius (1987). Gut clearance and pigment destruction in a herbivorous copepod, Acartia tonsa, and the determination of in situ grazing rates. Journal of Plankton Research, 9: 525-534. Roman, M.R. and P.A. Rublee (1981). A method to determine in situ grazing rates on natural particle assemblages. Marine Biology, 65: 303-309. Small, L.F., M.R. Landry, R.W. Eppley, F. Azam and A.F. Carlucci (1989). Role of plankton in the carbon and nitrogen budgets of the Santa Monica Basin, California. Marine Ecology Progress Series, 56: 57-74. Wiebe, P.H., A.W. Morton, A.M. Bradley, R.H. Backus, J.E. Craddock T.J. Cowles, V.A. Barber and G.R. Flierl (1985). New developments in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Marine Biology, 87: 313-323. |
TT045
| Website | |
| Platform | R/V Thomas G. Thompson |
| Start Date | 1995-03-14 |
| End Date | 1995-04-10 |
| Description | Methods & Sampling PI: Michael Roman of: Horn Point Environmental Laboratory dataset: Carbon biomass mesozooplankton, MOCNESS oblique tows dates: March 19, 1995 to April 06, 1995 location: N: 19.1451 S: 10.0063 W: 58.0007 E: 67.1653 project/cruise: Process 2 TN045 Inter-monsoon in the Arabian Sea ship: R/V Thomas Thompson Mesozooplankton Protocols Hans Dam and Michael Roman Mesozooplankton Biomass Mesozooplankton (> 64 �m) were collected from day/night pairs of tows with a 0.25 m-mouth area MOCNESS equipped with nine nets with a 7:1, mouth:length ratio (Wiebe et al., 1985). Transmission by underwater sensors on the MOCNESS through conducting cable to the deck yielded output of fluorescence, temperature, conductivity, depth, frame angle, volume filtered, and net closing response at 1-s intervals. Oblique tows were taken from 1000 m and 200 m to the surface. Eight depth strata were sampled on both shallow and deep tows: 0 - 10 m 0 - 20 m 10 - 20 m 20 - 40 m 20 - 40 m 40 - 100 m 40 - 60 m 100 - 200 m 60 - 80 m 200 - 400 m 80 - 100 m 400 - 600 m 100 - 150 m 600 - 800 m 150 - 200 m 800 - 1000 m Descent rates of the MOCNESS were generally between 40 and 50 m min and ascent speeds between 10 and 20 m min. The MOCNESS was towed at a ship speed of 2 kt which resulted in a net angle of near 45 . On board ship, the contents of each net were split in half with a Folsom Plankton Splitter. One half of the sample was preserved in 4% buffered (Sodium Borate) Formalin. The remaining half of the sample was gently wet-sieved through a 2.0 mm mesh to remove gelatinous zooplankton and micronekton (not caught quantitatively by 0.25 m MOCNESS). The portion passing through this mesh was wet-sieved further through 1.0 mm, 0.5 mm and 0.2 mm meshes. This procedure yielded four different size classes: 2.0-1.0 mm; 1.0-0.5 mm; 0.5-0.2 mm and 0.2-0.064 mm. The samples caught on these size fractions were diluted and thoroughly mixed in a known volume of filtered seawater and duplicate aliquots, drawn with a Hensen-Stempel pipette, filtered onto precombusted GF/D filters and rinsed with a small amount of distilled water to get rid of salt. Filters were dried at 60 C. Organic carbon and nitrogen for each filter were measured with a Model 440 Control Equipment CHN analyzer at the Horn Point Laboratory of the University of Maryland. The average error associated with subsampling the zooplankton catch for carbon analysis was 16% (standard deviation/mean). Zooplankton biomass of the different size fractions as well as the amount of total (> 64 �m) mesozooplankton is expressed as mg C m or mg C m(integrated). Processing Description Analysis methodology referenced in: Roman, M.R. et al, Zooplankton variability on the equator at 140W during the JGOFS EqPac Study. Deep Sea Research II, 42(2-3) : 673-693, 1995 Mesozooplankton Grazing - Isotope Method Measurements of grazing rates by the radioisotope uptake technique were done day and night, immediately following the MOCNESS tows, in short term, in situ incubations at 1, 10, 30, 50, 70 and 90 m (Roman and Rublee, 1981). Five-liter Plexiglas chambers (General Oceanics) with 64 µm-mesh covering the bottom were lowered 10 m past the desired depths and then raised to concentrate zooplankton in the chambers. A messenger triggered the close of the bottles and released the radioisotope tracers (25 µCi l of methylH-thymidine, > 75 Ci mmol and 50 µCi l Na CO, 55.0 Ci mmol ) into the chambers. After a 45-minute incubation on the hydrowire, the chambers were retrieved, zooplankton collected on nested 200 and 64 µm sieves. The zooplankton were rinsed (filtered seawater, 10% HCl and deionized water) onto preweighed 12 µm pore-size Nuclepore filters and dried. Using a dissecting microscope, visible detritus and phytoplankton were removed with a sable brush. The filters were weighed and the weight-specific dpm of the isotopes were measured. Zooplankton carbon was assumed to be 40% of their dry weight to calculate the dpm mg C of the zooplankton. The labelled particulate matter (< 64 µm) was collected on 0.2 µm and 2.0 µm pore-size Nuclepore filters to determine the specific activity of the particulate matter. We derived weight-specific corrections for the absorption and adsorption of the isotopes in shipboard experiments using both filtered seawater and time-0 controls. These corrections were generally less than 10% of experimental values. The isotope activity of the labelled particulate matter (> 2 µm) and zooplankton were used to calculate zooplankton filtration rates, F = liters filtered. mg zooplankton C h, after Daro (1978). The grazing impact of the zooplankton community, expressed as liters filtered m h, was calculated as the product of the weight-specific filtration rate determined from the in situ incubations and the zooplankton biomass in the same depth interval determined immediately prior to the grazing incubation. We used both C bicarbonate and [methyl] H thymidine to estimate the filtration rates of mesozooplankton upon autotrophs (phytoplankton and protozoa that consumed phytoplankton) and heterotrophs (free-living and attached bacteria and protozoa that have consumed bacteria). Mesozooplankton Grazing - Gut Fluorescence Method Zooplankton were collected day and night, immediately preceding MOCNESS tows, from vertical tows with opening-closing, 3/4 meter-Puget Sound nets equipped with 200 µm mesh and solid cod ends. Nets were towed to sample depth strata similar to those sampled by the MOCNESS in the upper 120 m. Three depth strata sampled were: 0-20 m, 20-60 m and 60-120 m. The contents of each net was immediately sieved under dim light red light to yield three size fractions: 2.0-1.0 mm; 1.0-0.5 mm and 0.5-0.2 mm. Each fraction was immediately filtered onto a glass fiber filter (GF/A) and frozen at -20 C. This procedure took about 5 min. Samples were analyzed in the laboratory ashore. We employed a modification of the procedure described by Dam and Peterson (1988). Thawed samples were placed under a dissecting scope, animals picked individually with jeweler's forceps without regard to species, gently rinsed with 0.2 µm-filtered seawater and placed in centrifuge tubes containing 90% high-grade, cold acetone solution. The whole procedure was performed under dim, red-light illumination. We typically took triplicate subsamples of 30-100 animals, with the number of individuals depending on the size fraction considered. A similar number of animals per sample was also picked for weight (C,N) determination. The tubes containing the acetone and animals were kept frozen at -20 C for at least 24 h for pigment extraction. The amount of pigment (chlorophyll and phaeopigments) per individual was measured fluorometrically and expressed in terms of chlorophyll (body carbon) (Dam et al., 1993). To convert gut pigment values to pigment ingestion rates, knowledge of the gut passage time is necessary. This is typically estimated from the reciprocal of the gut clearance rate constant (GCRC, units of time) of animals placed in filtered seawater (review in Dam and Peterson, 1988). GCRC estimated over the first 30-40 min of gut evacuation is not statistically different from the gut evacuation rate constant of feeding animals (Kiorboe and Tiselius, 1987; Ellis and Small, 1990). We estimated GCRC every time collections for gut pigment were made. To collect animals for gut evacuation experiments, one tow (upper 60 m) was done immediately after the ones for gut fluorescence. We estimated GCRC of animals in the different size fractions (0.2-0.5 mm; 0.5-1.0 mm; 1.0-2-0 mm) by employing the procedure described by Small et al. (1989); i.e., animals were quickly sorted into separate containers, kept in the dark in a temperature-controlled room. We monitored the decline in gut pigment, at 5-min. intervals, over the first 25 min of gut evacuation. Seventy-five per cent of the variance of GCRC is explained by temperature, with a Q of 2.2 (Dam and Peterson, 1988). Therefore, we corrected GCRC for differences in temperature from the depth at which animals were collected. Weight-specific ingestion rates for each depth layer were estimated from knowledge of gut pigment and GCRC. The grazing impact of zooplankton was estimated from the product of weight-specific ingestion rates and biomass for each size class. Literature Cited Dam, H.G., C.A. Miller and S.H. Jonasdottir (1993). The trophic role of mesozooplankton at 47 N, 20 W during the North Atlantic Bloom Experiment. Deep-Sea Research, 40: 197-212. Dam, H.G. and W.T. Peterson (1988). The effect of temperature on the gut clearance rate constant of planktonic copepods. Journal of Experimental Marine Biology and Ecology, 123: 1-14. Daro, M.H. (1978). A simplified C method for grazing measurements on natural planktonic populations. Helgolander wiss. Meeresunters, 31: 241-248. Ellis, S.G. and L.F. Small (1989). A comparison of gut evacuation rates of feeding and non-feeding Calanus marshallae. Marine Biology, 103: 175-181. Kiorboe, T. and P. Tiselius (1987). Gut clearance and pigment destruction in a herbivorous copepod, Acartia tonsa, and the determination of in situ grazing rates. Journal of Plankton Research, 9: 525-534. Roman, M.R. and P.A. Rublee (1981). A method to determine in situ grazing rates on natural particle assemblages. Marine Biology, 65: 303-309. Small, L.F., M.R. Landry, R.W. Eppley, F. Azam and A.F. Carlucci (1989). Role of plankton in the carbon and nitrogen budgets of the Santa Monica Basin, California. Marine Ecology Progress Series, 56: 57-74. Wiebe, P.H., A.W. Morton, A.M. Bradley, R.H. Backus, J.E. Craddock T.J. Cowles, V.A. Barber and G.R. Flierl (1985). New developments in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Marine Biology, 87: 313-323. |
TT050
| Website | |
| Platform | R/V Thomas G. Thompson |
| Start Date | 1995-08-18 |
| End Date | 1995-09-15 |
| Description | Methods & Sampling PI: Michael Roman of: Horn Point Environmental Laboratory dataset: Carbon biomass mesozooplankton, MOCNESS oblique tows dates: August 23, 1995 to September 11, 1995 location: N: 19.1776 S: 9.9603 W: 58.0268 E: 67.2369 project/cruise: Process 5 TN050 Summer monsoon in the Arabian Sea ship: R/V Thomas Thompson Mesozooplankton Protocols Hans Dam and Michael Roman Mesozooplankton Biomass Mesozooplankton (> 64 µm) were collected from day/night pairs of tows with a 0.25 m-mouth area MOCNESS equipped with nine nets with a 7:1, mouth:length ratio (Wiebe et al., 1985). Transmission by underwater sensors on the MOCNESS through conducting cable to the deck yielded output of fluorescence, temperature, conductivity, depth, frame angle, volume filtered, and net closing response at 1-s intervals. Oblique tows were taken from 1000 m and 200 m to the surface. Eight depth strata were sampled on both shallow and deep tows: 0 - 10 m 0 - 20 m 10 - 20 m 20 - 40 m 20 - 40 m 40 - 100 m 40 - 60 m 100 - 200 m 60 - 80 m 200 - 400 m 80 - 100 m 400 - 600 m 100 - 150 m 600 - 800 m 150 - 200 m 800 - 1000 m Descent rates of the MOCNESS were generally between 40 and 50 m min and ascent speeds between 10 and 20 m min. The MOCNESS was towed at a ship speed of 2 kt which resulted in a net angle of near 45 . On board ship, the contents of each net were split in half with a Folsom Plankton Splitter. One half of the sample was preserved in 4% buffered (Sodium Borate) Formalin. The remaining half of the sample was gently wet-sieved through a 2.0 mm mesh to remove gelatinous zooplankton and micronekton (not caught quantitatively by 0.25 m MOCNESS). The portion passing through this mesh was wet-sieved further through 1.0 mm, 0.5 mm and 0.2 mm meshes. This procedure yielded four different size classes: 2.0-1.0 mm; 1.0-0.5 mm; 0.5-0.2 mm and 0.2-0.064 mm. The samples caught on these size fractions were diluted and thoroughly mixed in a known volume of filtered seawater and duplicate aliquots, drawn with a Hensen-Stempel pipette, filtered onto precombusted GF/D filters and rinsed with a small amount of distilled water to get rid of salt. Filters were dried at 60 C. Organic carbon and nitrogen for each filter were measured with a Model 440 Control Equipment CHN analyzer at the Horn Point Laboratory of the University of Maryland. The average error associated with subsampling the zooplankton catch for carbon analysis was 16% (standard deviation/mean). Zooplankton biomass of the different size fractions as well as the amount of total (> 64 µm) mesozooplankton is expressed as mg C m or mg C m(integrated). Processing Description Analysis methodology referenced in: Roman, M.R. et al, Zooplankton variability on the equator at 140W during the JGOFS EqPac Study. Deep Sea Research II, 42(2-3) : 673-693, 1995 Mesozooplankton Grazing - Isotope Method Measurements of grazing rates by the radioisotope uptake technique were done day and night, immediately following the MOCNESS tows, in short term, in situ incubations at 1, 10, 30, 50, 70 and 90 m (Roman and Rublee, 1981). Five-liter Plexiglas chambers (General Oceanics) with 64 µm-mesh covering the bottom were lowered 10 m past the desired depths and then raised to concentrate zooplankton in the chambers. A messenger triggered the close of the bottles and released the radioisotope tracers (25 µCi l of methylH-thymidine, > 75 Ci mmol and 50 µCi l Na CO, 55.0 Ci mmol ) into the chambers. After a 45-minute incubation on the hydrowire, the chambers were retrieved, zooplankton collected on nested 200 and 64 µm sieves. The zooplankton were rinsed (filtered seawater, 10% HCl and deionized water) onto preweighed 12 µm pore-size Nuclepore filters and dried. Using a dissecting microscope, visible detritus and phytoplankton were removed with a sable brush. The filters were weighed and the weight-specific dpm of the isotopes were measured. Zooplankton carbon was assumed to be 40% of their dry weight to calculate the dpm mg C of the zooplankton. The labelled particulate matter (< 64 µm) was collected on 0.2 µm and 2.0 µm pore-size Nuclepore filters to determine the specific activity of the particulate matter. We derived weight-specific corrections for the absorption and adsorption of the isotopes in shipboard experiments using both filtered seawater and time-0 controls. These corrections were generally less than 10% of experimental values. The isotope activity of the labelled particulate matter (> 2 µm) and zooplankton were used to calculate zooplankton filtration rates, F = liters filtered. mg zooplankton C h, after Daro (1978). The grazing impact of the zooplankton community, expressed as liters filtered m h, was calculated as the product of the weight-specific filtration rate determined from the in situ incubations and the zooplankton biomass in the same depth interval determined immediately prior to the grazing incubation. We used both C bicarbonate and [methyl] H thymidine to estimate the filtration rates of mesozooplankton upon autotrophs (phytoplankton and protozoa that consumed phytoplankton) and heterotrophs (free-living and attached bacteria and protozoa that have consumed bacteria). Mesozooplankton Grazing - Gut Fluorescence Method Zooplankton were collected day and night, immediately preceding MOCNESS tows, from vertical tows with opening-closing, 3/4 meter-Puget Sound nets equipped with 200 µm mesh and solid cod ends. Nets were towed to sample depth strata similar to those sampled by the MOCNESS in the upper 120 m. Three depth strata sampled were: 0-20 m, 20-60 m and 60-120 m. The contents of each net was immediately sieved under dim light red light to yield three size fractions: 2.0-1.0 mm; 1.0-0.5 mm and 0.5-0.2 mm. Each fraction was immediately filtered onto a glass fiber filter (GF/A) and frozen at -20 C. This procedure took about 5 min. Samples were analyzed in the laboratory ashore. We employed a modification of the procedure described by Dam and Peterson (1988). Thawed samples were placed under a dissecting scope, animals picked individually with jeweler's forceps without regard to species, gently rinsed with 0.2 µm-filtered seawater and placed in centrifuge tubes containing 90% high-grade, cold acetone solution. The whole procedure was performed under dim, red-light illumination. We typically took triplicate subsamples of 30-100 animals, with the number of individuals depending on the size fraction considered. A similar number of animals per sample was also picked for weight (C,N) determination. The tubes containing the acetone and animals were kept frozen at -20 C for at least 24 h for pigment extraction. The amount of pigment (chlorophyll and phaeopigments) per individual was measured fluorometrically and expressed in terms of chlorophyll (body carbon) (Dam et al., 1993). To convert gut pigment values to pigment ingestion rates, knowledge of the gut passage time is necessary. This is typically estimated from the reciprocal of the gut clearance rate constant (GCRC, units of time) of animals placed in filtered seawater (review in Dam and Peterson, 1988). GCRC estimated over the first 30-40 min of gut evacuation is not statistically different from the gut evacuation rate constant of feeding animals (Kiorboe and Tiselius, 1987; Ellis and Small, 1990). We estimated GCRC every time collections for gut pigment were made. To collect animals for gut evacuation experiments, one tow (upper 60 m) was done immediately after the ones for gut fluorescence. We estimated GCRC of animals in the different size fractions (0.2-0.5 mm; 0.5-1.0 mm; 1.0-2-0 mm) by employing the procedure described by Small et al. (1989); i.e., animals were quickly sorted into separate containers, kept in the dark in a temperature-controlled room. We monitored the decline in gut pigment, at 5-min. intervals, over the first 25 min of gut evacuation. Seventy-five per cent of the variance of GCRC is explained by temperature, with a Q of 2.2 (Dam and Peterson, 1988). Therefore, we corrected GCRC for differences in temperature from the depth at which animals were collected. Weight-specific ingestion rates for each depth layer were estimated from knowledge of gut pigment and GCRC. The grazing impact of zooplankton was estimated from the product of weight-specific ingestion rates and biomass for each size class. Literature Cited Dam, H.G., C.A. Miller and S.H. Jonasdottir (1993). The trophic role of mesozooplankton at 47 N, 20 W during the North Atlantic Bloom Experiment. Deep-Sea Research, 40: 197-212. Dam, H.G. and W.T. Peterson (1988). The effect of temperature on the gut clearance rate constant of planktonic copepods. Journal of Experimental Marine Biology and Ecology, 123: 1-14. Daro, M.H. (1978). A simplified C method for grazing measurements on natural planktonic populations. Helgolander wiss. Meeresunters, 31: 241-248. Ellis, S.G. and L.F. Small (1989). A comparison of gut evacuation rates of feeding and non-feeding Calanus marshallae. Marine Biology, 103: 175-181. Kiorboe, T. and P. Tiselius (1987). Gut clearance and pigment destruction in a herbivorous copepod, Acartia tonsa, and the determination of in situ grazing rates. Journal of Plankton Research, 9: 525-534. Roman, M.R. and P.A. Rublee (1981). A method to determine in situ grazing rates on natural particle assemblages. Marine Biology, 65: 303-309. Small, L.F., M.R. Landry, R.W. Eppley, F. Azam and A.F. Carlucci (1989). Role of plankton in the carbon and nitrogen budgets of the Santa Monica Basin, California. Marine Ecology Progress Series, 56: 57-74. Wiebe, P.H., A.W. Morton, A.M. Bradley, R.H. Backus, J.E. Craddock T.J. Cowles, V.A. Barber and G.R. Flierl (1985). New developments in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Marine Biology, 87: 313-323. |
U.S. JGOFS Arabian Sea (Arabian Sea)
The U.S. Arabian Sea Expedition which began in September 1994 and ended in January 1996, had three major components: a U.S. JGOFS Process Study, supported by the National Science Foundation (NSF); Forced Upper Ocean Dynamics, an Office of Naval Research (ONR) initiative; and shipboard and aircraft measurements supported by the National Aeronautics and Space Administration (NASA). The Expedition consisted of 17 cruises aboard the R/V Thomas Thompson, year-long moored deployments of five instrumented surface buoys and five sediment-trap arrays, aircraft overflights and satellite observations. Of the seventeen ship cruises, six were allocated to repeat process survey cruises, four to SeaSoar mapping cruises, six to mooring and benthic work, and a single calibration cruise which was essentially conducted in transit to the Arabian Sea.
U.S. Joint Global Ocean Flux Study (U.S. JGOFS)
The United States Joint Global Ocean Flux Study was a national component of international JGOFS and an integral part of global climate change research.
The U.S. launched the Joint Global Ocean Flux Study (JGOFS) in the late 1980s to study the ocean carbon cycle. An ambitious goal was set to understand the controls on the concentrations and fluxes of carbon and associated nutrients in the ocean. A new field of ocean biogeochemistry emerged with an emphasis on quality measurements of carbon system parameters and interdisciplinary field studies of the biological, chemical and physical process which control the ocean carbon cycle. As we studied ocean biogeochemistry, we learned that our simple views of carbon uptake and transport were severely limited, and a new "wave" of ocean science was born. U.S. JGOFS has been supported primarily by the U.S. National Science Foundation in collaboration with the National Oceanic and Atmospheric Administration, the National Aeronautics and Space Administration, the Department of Energy and the Office of Naval Research. U.S. JGOFS, ended in 2005 with the conclusion of the Synthesis and Modeling Project (SMP).
| Funding Source | Award |
|---|---|
| National Science Foundation (NSF) |
[ table of contents | back to top ]