Dissolved Pb passing through a 0.2 um Acropak capsule filter from R/V Thomas G. Thompson cruise TN303 in the Eastern Tropical Pacific in 2013 (U.S. GEOTRACES EPZT project)
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Boyle, Edward A. | Massachusetts Institute of Technology (MIT-EAPS) | Principal Investigator, Contact |
| Lee, Jong-Mi | University of California-Santa Cruz (UCSC) | Co-Principal Investigator |
| Zurbrick, Cheryl | Massachusetts Institute of Technology (MIT-EAPS) | Co-Principal Investigator |
| Rauch, Shannon | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Abstract
Dissolved Pb passing through a 0.2 um Acropak capsule filter. Samples were collected on the US GEOTRACES East Pacific Zonal Transect (EPZT) cruise in 2013.
Sample storage bottle lids and threads were soaked overnight in 2N reagent grade HCl, then filled with 1N reagent grade HCl to be heated in an oven at 60 degrees C overnight, inverted, heated for a second day, and rinsed 5X with pure distilled water. The bottles were then filled with trace metal clean dilute HCl (~0.01N HCl) and again heated in the oven for one day on either end. Clean sample bottles were emptied, and double-bagged prior to rinsing and filling with sample.
Trace metal-clean seawater samples were collected using the U.S. GEOTRACES sampling system consisting of 24 Teflon-coated GO-FLO bottles that had been pre-rinsed with a 24+ hour treatment of filtered surface seawater at the beginning of the cruise (see Cutter & Bruland, 2012 for more information on the sampling system). At each station, the bottles were deployed open and tripped on ascent at 3 m/min. On deck, the bottles were kept in a trace metal clean sampling van over-pressurized with HEPA-filtered air, except immediately prior to and following deployments, in which cases they were covered on both ends with shower caps to avoid deck contamination.
During sampling in the clean van, the GO-FLO bottles were pressurized to ~0.4 atm with HEPA-filtered air, and their spigots were fitted with an acid-cleaned piece of Bev-a-Line tubing that fed into an Acropak-200 Supor capsule filter (0.2 um pore size made of polyethersulfone). Before use, this filter had been filled with filtered surface seawater that had been acidified to pH 2 with trace metal clean HCl and left overnight to rinse. Before collecting any subsamples, at least 500 mL of seawater was passed through the filter (while eliminating air bubbles in the capsule reservoir). Subsamples were taken into acid-cleaned (see above) Nalgene HDPE bottles after a triple rinse with the sample. Acropak filters were used for at most 3 casts before a new filter was used, and they were stored empty in a refrigerator while not in use. GEOFish surface samples were taken using an all-plastic "towed fish" pumping system as described in Bruland et al. 2005 at approximately 3 m depth and were subsampled identically.
All samples were acidified back in the Boyle laboratory at 2 mL per liter seawater (pH ~2) with trace metal clean 6N HCl.
Samples were analyzed at least 1 month after acidification over 52 analytical sessions by one of two methods: a resin pre-concentration or magnesium hydroxide co-precipitation for very low level Pb concentration analyses. Details of the two methods are as follows:
(1) Resin pre-concentration: This method utilized the isotope-dilution ICP-MS method described in Lee et al. 2011, which includes pre-concentration on nitrilotriacetate (NTA) resin and analysis on a Fisons PQ2+ using a 400 uL/min nebulizer. Briefly, samples were poured into 30 mL subsample bottles. Then, triplicate 1.5 mL polyethylene vials (Eppendorf AG) were rinsed three times with the 30 mL subsample. Each sample was pipetted (1.3 mL) from the 30 mL subsample to the 1.5 mL vial. Pipettes were calibrated daily to the desired volume. 25uL of a 204Pb spike were added to each sample, and the pH was raised to 5.3 using a trace metal clean ammonium acetate buffer, prepared at a pH of between 7.9 and 7.98. ~2400 beads of NTA Superflow resin (Qiagen Inc., Valencia, CA) were added to the mixture, and the vials were set to shake on a shaker for 4 days to allow the sample to equilibrate with the resin. After 4 days, the beads were centrifuged and washed 3 times with pure distilled water, using a trace metal clean syphon tip to remove the water wash from the sample vial following centrifugation. After the last wash, 320 uL of a 0.1N solution of trace metal clean HNO3 was added to the resin to elute the metals, and the samples were set to shake on a shaker for 2 days prior to analysis by ICP-MS. NTA Superflow resin was cleaned by batch rinsing with 0.1N trace metal clean HCl for a few hours, followed by multiple washes until the pH of the solution was above 4. Resin was stored at 4 degrees C in the dark until use, though it was allowed to equilibrate to room temperature prior to the addition to the sample. Eppendorf polyethylene vials were cleaned by heated submersion for 2 days at 60 degrees C in 1N reagent grade HCl, followed by a bulk rinse and 4X individual rinse of each vial with pure distilled water. Each vial was then filled with trace metal clean dilute HCl (~0.01N HCl) and heated in the oven at 60 degrees C for one day on either end. Vials were kept filled until just before usage.
(2) Magnesium hydroxide co-precipitation (a.k.a “Low level method”): This method works for all seawater samples, but was employed especially for lower level Pb concentration samples. The method is a lower volume seawater adaptation of the isotope ratio method of Reuer et al., 2003, which includes pre-concentration by co-precipitation and analysis on a Fisons PQ2+ using a 400 uL/min nebulizer. Briefly, 50 mL polypropylene conical centrifuge tubes (Corning) were weighed and recorded before rinsing three times with seawater sample or blank (low trace metal seawater) and filling with ~40 mL of sample (10 mL of blank). Samples were processed in duplicate. Each sample vial was then weighed and recorded for an accurate measurement of sample volume by mass. Each sample and blank was then spiked with 100 uL of a 204Pb spike; pipettes were calibrated daily to the desired volume. After a 10 – 60 minute equilibration period, trace metal clean ammonium hydroxide, prepared at a pH of between 8.5 and 9.5 was added such that co-precipitation would occur within 8 hours. Samples were left to precipitate for 12 – 24 hours prior to centrifuging, siphoning off of seawater using a trace metal clean siphon tip, and dissolution in 50 – 100 uL trace metal clean HCl (6M), determined visually for each sample. Samples were then re-precipitated with 20 – 50 uL of ammonium hydroxide (instantaneous) and left to sit for 12 – 24 hours prior to centrifuging, siphoning off of seawater using a trace metal clean siphon tip, and dissolution in 200 – 500 uL trace metal clean HBr (1.1M). Excess salts in the samples were then removed by processing through an anion exchange column chemistry based on Krogh, 1973 and Manhesa et al., 1978. In brief, trace metal cleaned Eichrom AG-1x8 resin was used with in-house made mini-Teflon columns; resin was cleaned with 2mL HCl (6M), equilibrated with 1mL HBr (1.1M); samples were loaded (in 1.1M HBr), washed with 1.5 mL HBr (1.1M), washed with 0.8 mL HCl (2M) and eluted for collection with 2 mL HCl (6M). Concentrated samples were evaporated to dryness until analysis. Prior to analysis, samples were dissolved in 500 uL of 0.1M trace metal clean HNO3. For each set of samples, three low trace metal seawater blanks were concurrently extracted; for each column separation, 1 chemistry blank was run. Eichrom AG-1x8 resin was cleaned by three batch rinses with 6N trace metal clean HCl for a ~12 hours on a shaker table, followed by multiple washes with distilled water until the pH of the solution was above 4.5. Resin was stored at room temperature in the dark until use. Corning polypropylene conical centrifuge tubes (50mL) were cleaned by heated submersion for 2 days at 60 degrees C in 1N reagent grade HCl, followed by a bulk rinse and 4X individual rinse of each vial with pure distilled water. Each vial was then filled with trace metal clean dilute HCl (~0.01N HCl) and heated in the oven at 60 degrees C for one day on either end. Vials were kept filled until just before usage.
On each day of sample analysis, procedure blanks were determined. For the resin preconcentration method, 12 replicates of 300 uL of an in-house standard reference material seawater (low Pb surface water) were used, where the amount of Pb in the 300 uL was verified as negligible. For the "low level" magnesium hydroxide co-precipitation method, 3 replicates of ~10 mL of the same in-house standard reference material seawater were used. The procedural blank over the relevant sessions for resin preconcentration method ranged from 2.2-7.3 pmol/kg, averaging 4.9 ± 1.4 pmol/kg; for the magnesium hydroxide co-precipitation method, blanks ranged from 0.2 – 3.9 pmol/kg, averaging 1.3 ± 0.9 pmol/kg. Within a day, procedure blanks were very reproducible with an average standard deviation of 0.8 pmol/kg (resin preconcentration method) and 0.3 pmol/kg (magnesium hydroxide co-precipitation method), resulting in detection limits (3x this standard deviation) of 2.4 pmol/kg and 0.9 pmol/kg. Replicate analyses of three different large-volume seawater samples (one with ~13 pmol/kg, another with ~24 pmol/kg, and a third with ~35 pmol/kg) indicated that the precision of the analysis is similar for both methods: 4% or 1.2 pmol/kg, whichever is larger, for the resin preconcentration method; 10% or 0.9 pmol/kg, whichever is larger for the "low level" method.
Triplicate analyses of an international reference standard gave SAFe D2: 27.2 ± 1.7 pmol/kg. However, this standard run was linked into our own long-term quality control standards that are run on every analytical day to maintain long-term consistency.
The Pb concentrations of these samples were quite low, so in general the errors are determined more by uncertainty in the blank corrections rather than as a fixed percentage of the concentration. Profile samples were intercalibrated with Dr. Ken Bruland and his student Claire Parker and Dr. Russ Flegal and his technician Ralph Till, who analyzed separate samples from USGT-EPZT stations, and each used different analytical schemes. The standard deviation of the concentration differences for 213 GoFlo samples (excluding 5 samples with lab to lab differences of >5 pmol/kg) analyzed by MIT and the UCSC Bruland lab was 1.5 pmol/kg. The standard deviation of the concentration differences for 77 GoFlo samples (excluding 5 samples with lab to lab differences of >5 pmol/kg) analyzed by the UCSC-Flegal lab and the UCSC Bruland lab was 1.6 pmol/kg. We therefore believe that data from all three labs for this cruise are comparable to about 1.6 pmol/kg (one sigma). Analyses from an individual lab relative to other data from the same lab for this cruise are comparable to that lab’s standard deviation on standards or samples near the detection limits. For the MIT lab, the pooled standard deviation of 17 samples near the detection limit (analyzed by the "low level" method) for station 30 was 0.4 pmol/kg.
One third of the MIT samples were analyzed by Jong-Mi Lee using the resin preconcentration method, and the remaining two-thirds of the cruise samples were analyzed by Cheryl Zurbrick using both the resin preconcentration and "low level" methods. There was no significant difference between them for the lowest concentration large-volume seawater reference sample (JML averaged 13.2 ± 0.2 pmol/kg; CMZ averaged 13.3 ± 1.3 pmol/kg).
Samples were analyzed in triplicate (resin preconcentration method) or duplicate (magnesium hydroxide co-precipitation method), and average values shown in this database were only accepted if reproduced over at least two replicates; otherwise, the data was re-analyzed until a reproducible value was reached.
The standard Ocean Data View flags were used (reference all flags at https://www.bodc.ac.uk/data/codes_and_formats/odv_format/):
1: Good Value: Good quality data value that is not part of any identified malfunction and has been verified as consistent with real phenomena during the quality control process.
2: Probably Good Value: Data value that is probably consistent with real phenomena but this is unconfirmed or data value forming part of a malfunction that is considered too small to affect the overall quality of the data object of which it is a part. [Used when only one replicate confirmed the reported value.]
3: Probably Bad Value: Data value recognized as unusual during quality control that forms part of a feature that is probably inconsistent with real phenomena.
4: Bad Value: An obviously erroneous data value.
6: Value Below Detection Limit: The level of the measured phenomenon was too small to be quantified by the technique employed to measure it. The accompanying value is the detection limit for the technique or zero if that value is unknown.
9: Missing Value: The data value is missing. Any accompanying value will be a magic number representing absent data. [All samples collected were analyzed. Any missing data was not collected.]
In this dataset, we did not encounter any samples that did not yield acceptably reproducible results upon repeated analysis, so we believe that the data truly represents the concentration of Pb in the sample collection bottle. However, there were a few points that were high based on adjacent samples and for which an obvious hydrographic argument could not be made for the anomaly. These samples may be contaminated, and they are given the quality control flag of 3 (probably bad value).
BCO-DMO Processing:
- modified parameter names to conform with BCO-DMO and GEOTRACES naming conventions;
- replaced blanks with "nd" (no data);
- moved GeoFish data into separate columns per GEOTRACES naming conventions;
- made corrections in data values to two samples (8792 and 9865) per request from PI (July 13, 2016);
- made corrections to quality flags for 3 samples (8375, 8377, 8379) per request from PI (July 14, 2016).
Additional GEOTRACES Processing by BCO-DMO:
As was done for the GEOTRACES-NAT data, BCO-DMO added standard US GEOTRACES information, such as the US GEOTRACES event number, to each submitted dataset lacking this information. To accomplish this, BCO-DMO compiled a 'master' dataset composed of the following parameters:
cruise_id, EXPOCODE,SECT_ID, STNNBR, CASTNO, GEOTRC_EVENTNO, GEOTRC_SAMPNO, GEOTRC_INSTR, SAMPNO, GF_NO, BTLNBR, BTLNBR_FLAG_W, DATE_START_EVENT, TIME_START_EVENT, ISO_DATETIME_UTC_START_EVENT, EVENT_LAT, EVENT_LON, DEPTH_MIN, DEPTH_MAX, BTL_DATE, BTL_TIME, BTL_ISO_DATETIME_UTC, BTL_LAT, BTL_LON, ODF_CTDPRS, SMDEPTH, FMDEPTH, BTMDEPTH, CTDPRS, CTDDEPTH.
This added information will facilitate subsequent analysis and inter-comparison of the datasets.
Bottle parameters in the master file were taken from the GT-C_Bottle and ODF_Bottle datasets. Non-bottle parameters, including those from GeoFish tows, Aerosol sampling, and McLane Pumps, were taken from the TN303 Event Log (version 30 Oct 2014). Where applicable, pump information was taken from the PUMP_Nuts_Sals dataset.
A standardized BCO-DMO method (called "join") was then used to merge the missing parameters to each US GEOTRACES dataset, most often by matching on sample_GEOTRC or on some unique combination of other parameters.
If the master parameters were included in the original data file and the values did not differ from the master file, the original data columns were retained and the names of the parameters were changed from the PI-submitted names to the standardized master names. If there were differences between the PI-supplied parameter values and those in the master file, both columns were retained. If the original data submission included all of the master parameters, no additional columns were added, but parameter names were modified to match the naming conventions of the master file.
See the dataset parameters documentation for a description of which parameters were supplied by the PI and which were added via the join method.
| File |
|---|
Pb_joined.csv (Comma Separated Values (.csv), 114.78 KB) MD5:8b6d97b0b5d6ccdead3924ce6993824b Primary data file for dataset ID 644607 |
| Parameter | Description | Units |
| cruise_id | Cruise identifier. TN = R/V Thomas G. Thompson. | unitless |
| SECT_ID | GEOTRACES cruise name. | unitless |
| EXPOCODE | Cruise EXPO code. | unitless |
| STNNBR | Station number. | unitless |
| GEOTRC_EVENTNO | GEOTRACES event number. | unitless |
| date_pi | Sampling date, provided by the PI; in YYYYmmdd format. | unitless |
| time_pi | Sampling time, provided by the PI; in HHMM format. | unitless |
| lat_pi | Latitude where sample was collected, provided by the PI. | decimal degrees |
| lon_pi | Longitude where sample was collected, provided by the PI. | decimal degrees |
| BTMDEPTH | Bottom depth, provided by PI. | meters |
| ISO_DATETIME_UTC_START_EVENT | Date and time (UTC) at start of the event, formatted to ISO 8601 standard, according to the event log. | YYYY-mm-ddTHH:MM:SS.xxZ |
| EVENT_LAT | Latitude at the start of the event, according to the event log. | decimal degrees |
| EVENT_LON | Longitude at the start of the event, according to the event log. | decimal degrees |
| GEOTRC_SAMPNO | GEOTRACES sample number. | dimensionless |
| CTDPRS | CTD pressure, according to bottle file. | decibars |
| depth_pi | Sampling depth, provided by the PI. | meters |
| Pb_D_CONC_BOTTLE | Dissolved Pb, the Pb passing through a 0.2 um Acropak capsule filter, in picomoles Pb per kg of seawater (sample size was quantified volumetrically at room temperature, then converted to weight units assuming a density of 1.027 g/cc); samples collected by GT-C rosette. | picomoles per kilogram (pmol/kg) |
| Pb_D_CONC_BOTTLE_FLAG_W | Quality flag for Pb_D_CONC_BOTTLE. See "Processing Description" for flag definitions. | unitless |
| N | Number of samples (N). | dimensionless |
| Pb_D_CONC_BOTTLE_STDEV | Standard deviation of Pb_D_CONC_BOTTLE. | picomoles per kilogram (pmol/kg) |
| Pb_D_CONC_FISH | Dissolved Pb, the Pb passing through a 0.2 um Acropak capsule filter, in picomoles Pb per kg of seawater (sample size was quantified volumetrically at room temperature, then converted to weight units assuming a density of 1.027 g/cc); samples collected by GeoFish. | picomoles per kilogram (pmol/kg) |
| Pb_D_CONC_FISH_FLAG_W | Quality flag for Pb_D_CONC_FISH. See "Processing Description" for flag definitions. | unitless |
| Pb_D_CONC_FISH_STDEV | Standard deviation of Pb_D_CONC_FISH. | picomoles per kilogram (pmol/kg) |
| CASTNO | Cast number. | unitless |
| SAMPNO | Bottle sample number. | unitless |
| BTLNBR | Bottle number. | unitless |
| BTLNBR_FLAG_W | WOCE bottle quality flag. | unitless |
| GEOTRC_INSTR | Sampling instrument. | unitless |
| GFISH_NO | GEOTRACES towfish ("GeoFish") number. | unitless |
| BTL_ISO_DATETIME_UTC | Date and time (UTC) when the bottle was fired, formatted to ISO 8601 standard, according to the bottle file. | YYYY-mm-ddTHH:MM:SS.xxZ |
| BTL_LAT | Latitude when the bottle was fired, according to the bottle file. | decimal degrees |
| BTL_LON | Longitude when the bottle was fired, according to the bottle file. | decimal degrees |
| ODF_CTDPRS | Pressure, determined by ODF group. | decibars |
| SMDEPTH | SMDEPTH is Saunders-Mantyla depth (integrated; uses dynamic height) | meters |
| FMDEPTH | FMDEPTH is Fofonoff-Millard depth (non-integrated; also used by SBE) | meters |
| CTDDEPTH | CTD depth, provided by the PI. | meters |
| Dataset-specific Instrument Name | Teflon-coated GO-FLO bottles |
| Generic Instrument Name | GO-FLO Teflon Trace Metal Bottle |
| Generic Instrument Description | GO-FLO Teflon-lined Trace Metal free sampling bottles are used for collecting water samples for trace metal, nutrient and pigment analysis. The GO-FLO sampling bottle is designed specifically to avoid sample contamination at the surface, internal spring contamination, loss of sample on deck (internal seals), and exchange of water from different depths. |
| Dataset-specific Instrument Name | ICP-MS |
| Generic Instrument Name | Inductively Coupled Plasma Mass Spectrometer |
| Generic Instrument Description | An ICP Mass Spec is an instrument that passes nebulized samples into an inductively-coupled gas plasma (8-10000 K) where they are atomized and ionized. Ions of specific mass-to-charge ratios are quantified in a quadrupole mass spectrometer. |
TN303
| Website | |
| Platform | R/V Thomas G. Thompson |
| Report | |
| Start Date | 2013-10-25 |
| End Date | 2013-12-20 |
| Description | A zonal transect in the eastern tropical South Pacific (ETSP) from Peru to Tahiti as the second cruise of the U.S.GEOTRACES Program. This Pacific section includes a large area characterized by high rates of primary production and particle export in the eastern boundary associated with the Peru Upwelling, a large oxygen minimum zone that is a major global sink for fixed nitrogen, and a large hydrothermal plume arising from the East Pacific Rise. This particular section was selected as a result of open planning workshops in 2007 and 2008, with a final recommendation made by the U.S.GEOTRACES Steering Committee in 2009. It is the first part of a two-stage plan that will include a meridional section of the Pacific from Tahiti to Alaska as a subsequent expedition.
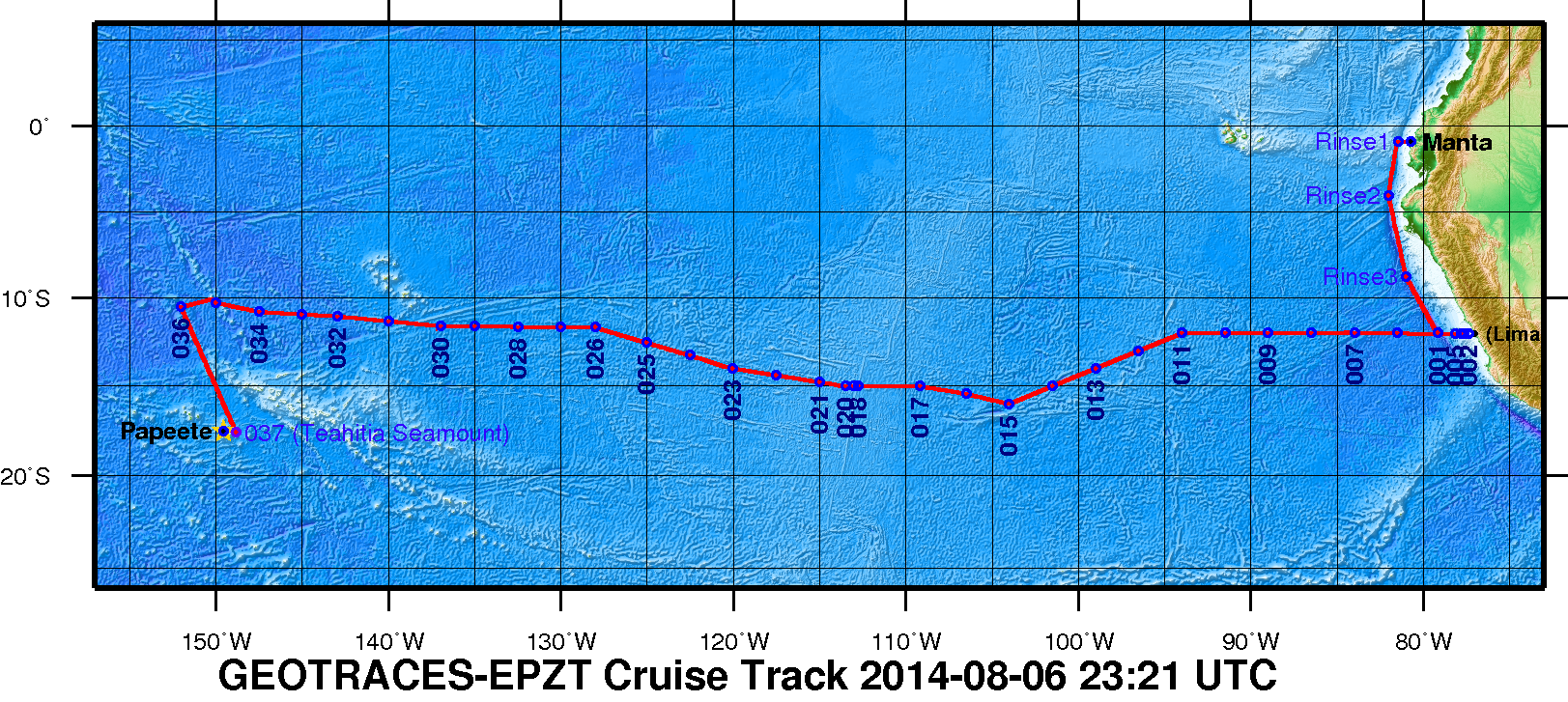
Figure 1. The 2013 GEOTRACES EPZT Cruise Track. [click on the image to view a larger version]
Additional cruise information is available from the Rolling Deck to Repository (R2R): http://www.rvdata.us/catalog/TN303 |
U.S. GEOTRACES East Pacific Zonal Transect (GP16) (U.S. GEOTRACES EPZT)
From the NSF Award Abstract
The mission of the International GEOTRACES Program (https://www.geotraces.org/), of which the U.S. chemical oceanography research community is a founding member, is "to identify processes and quantify fluxes that control the distributions of key trace elements and isotopes in the ocean, and to establish the sensitivity of these distributions to changing environmental conditions" (GEOTRACES Science Plan, 2006). In the United States, ocean chemists are currently in the process of organizing a zonal transect in the eastern tropical South Pacific (ETSP) from Peru to Tahiti as the second cruise of the U.S.GEOTRACES Program. This Pacific section includes a large area characterized by high rates of primary production and particle export in the eastern boundary associated with the Peru Upwelling, a large oxygen minimum zone that is a major global sink for fixed nitrogen, and a large hydrothermal plume arising from the East Pacific Rise. This particular section was selected as a result of open planning workshops in 2007 and 2008, with a final recommendation made by the U.S.GEOTRACES Steering Committee in 2009. It is the first part of a two-stage plan that will include a meridional section of the Pacific from Tahiti to Alaska as a subsequent expedition.
This award provides funding for management of the U.S.GEOTRACES Pacific campaign to a team of scientists from the University of Southern California, Old Dominion University, and the Woods Hole Oceanographic Institution. The three co-leaders will provide mission leadership, essential support services, and management structure for acquiring the trace elements and isotopes samples listed as core parameters in the International GEOTRACES Science Plan, plus hydrographic and nutrient data needed by participating investigators. With this support from NSF, the management team will (1) plan and coordinate the 52-day Pacific research cruise described above; (2) obtain representative samples for a wide variety of trace metals of interest using conventional CTD/rosette and GEOTRACES Sampling Systems; (3) acquire conventional JGOFS/WOCE-quality hydrographic data (CTD, transmissometer, fluorometer, oxygen sensor, etc) along with discrete samples for salinity, dissolved oxygen (to 1 uM detection limits), plant pigments, redox tracers such as ammonium and nitrite, and dissolved nutrients at micro- and nanomolar levels; (4) ensure that proper QA/QC protocols are followed and reported, as well as fulfilling all GEOTRACES Intercalibration protocols; (5) prepare and deliver all hydrographic-type data to the GEOTRACES Data Center (and US data centers); and (6) coordinate cruise communications between all participating investigators, including preparation of a hydrographic report/publication.
Broader Impacts: The project is part of an international collaborative program that has forged strong partnerships in the intercalibration and implementation phases that are unprecedented in chemical oceanography. The science product of these collective missions will enhance our ability to understand how to interpret the chemical composition of the ocean, and interpret how climate change will affect ocean chemistry. Partnerships include contributions to the infrastructure of developing nations with overlapping interests in the study area, in this case Peru. There is a strong educational component to the program, with many Ph.D. students carrying out thesis research within the program.
Figure 1. The 2013 GEOTRACES EPZT Cruise Track. [click on the image to view a larger version]
Collaborative Research: GEOTRACES Pacific section: Spatial variability of lead concentrations and isotopic compositions in the Eastern Tropical South Pacific (EPZT Pb)
Description from NSF award abstract:
Scientists from Massachusetts Institute of Technology and the University of California, Santa Cruz, plan to measure lead (Pb) concentrations and Pb isotopes (204Pb, 206Pb, 207Pb and 208Pb) in seawater profiles, aerosols, and hydrothermal plume samples collected during the 2013 GEOTRACES cruise from Peru to Tahiti.
Not only will the data significantly add to the limited data set existing for the South Pacific Ocean, results will be used to further our knowledge on the cycling of this element in the marine environment, as well as improve our understanding of anthropogenic inputs of Pb to the region of interest which is important given increases in lead emissions from mining, smelting, and fossil fuel combustion. In addition, data from the East Pacific Rise plume will document whether hydrothermal vent systems contribute to the oceanic Pb budget.
U.S. GEOTRACES (U.S. GEOTRACES)
GEOTRACES is a SCOR sponsored program; and funding for program infrastructure development is provided by the U.S. National Science Foundation.
GEOTRACES gained momentum following a special symposium, S02: Biogeochemical cycling of trace elements and isotopes in the ocean and applications to constrain contemporary marine processes (GEOSECS II), at a 2003 Goldschmidt meeting convened in Japan. The GEOSECS II acronym referred to the Geochemical Ocean Section Studies To determine full water column distributions of selected trace elements and isotopes, including their concentration, chemical speciation, and physical form, along a sufficient number of sections in each ocean basin to establish the principal relationships between these distributions and with more traditional hydrographic parameters;
* To evaluate the sources, sinks, and internal cycling of these species and thereby characterize more completely the physical, chemical and biological processes regulating their distributions, and the sensitivity of these processes to global change; and
* To understand the processes that control the concentrations of geochemical species used for proxies of the past environment, both in the water column and in the substrates that reflect the water column.
GEOTRACES will be global in scope, consisting of ocean sections complemented by regional process studies. Sections and process studies will combine fieldwork, laboratory experiments and modelling. Beyond realizing the scientific objectives identified above, a natural outcome of this work will be to build a community of marine scientists who understand the processes regulating trace element cycles sufficiently well to exploit this knowledge reliably in future interdisciplinary studies.
Expand "Projects" below for information about and data resulting from individual US GEOTRACES research projects.
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]