Apparent Quantum Yields for the Photochemical Formation of Carbonyl Compounds in Seawater from the R/V Endeavor EN589 in the Northwest Atlantic Ocean from September to October 2016.
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Kieber, David J. | State University of New York College of Environmental Science and Forestry (SUNY ESF) | Principal Investigator |
| Zhu, Yuting | State University of New York College of Environmental Science and Forestry (SUNY ESF) | Contact |
| Soenen, Karen | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Abstract
Apparent Quantum Yields for the Photochemical Formation of Carbonyl Compounds in Seawater from the R/V Endeavor EN589 in the Northwest Atlantic Ocean from September to October 2016.
Seawater sampling and Experimental Set up. Seawater was gravity filtered directly from the Niskin bottle through a 0.2-μm POLYCAP 75 AS Nylon filter (Whatman) into two 2 L Qorpak glass bottles previously rinsed by Milli Q water and muffled at 550 ˚C for 8 h. Each bottle was filled leaving 3–5 mL headspace, sealed with a Teflon-lined silicone screw cap, and stored at 4 °C in the dark until analyzed in Syracuse, NY. POLYCAP filters were cleaned prior to use by alternating rinses of acetonitrile and Milli Q water followed by extensive flushing with Milli Q. Prior to irradiation experiments, a Milli Q sample or 0.2 μm-filtered seawater sample was pneumatically pushed through 1/8” O.D. Teflon tubing with ultra-high purity helium (99.999%) into a rectangular quartz cell (4 mL capacity, 1 cm pathlength, Spectrocell, Inc.) for at least 10 min at a flow rate of 2 mL min-1. The quartz cell was periodically inverted to remove residual air bubbles. The quartz cell was sealed with a screw cap containing a Teflon-lined silicone septum insert. Once the quartz cell was filled with a sample, it was placed into an enclosed temperature-controlled cell holder equipped with a stirrer. All irradiations were performed using a model QP-SX10001, 1000 W xenon lamp (Superior Quartz Products, Inc.) along with a GM 252 monochromator (Spectral Energy, Corp.). A 10 nm bandwidth was used for irradiations <330 nm and a 20 nm bandwidth was used for longer wavelengths. A longpass filter with a 307 nm cutoff (50% transmission at 307 nm) was placed in the optical path directly after the monochromator for wavelengths >330 nm. Irradiation times varied from 1 to 36 h and were chosen depending on the wavelength of the irradiation and the absorbance of the seawater sample. Dark controls were incubated in the cell holder for up to 36 h. Except when noted, the cell holder temperature was set at 20 °C for all irradiations.
Carbonyl Determination. A 2.2 mL aliquot of the irradiated seawater sample or dark control was added to a 20 L aliquot of the 2,4-dinitrophenylhydrazine (DNPH) reagent in a ~2.2 mL Qorpak vial that was capped tightly with no headspace. The lid of the cap contained a Teflon-lined silicone septum. All samples were reacted at room temperature for a minimum of 12 h and a maximum of 48 h. Derivatized standards (Sigma-Aldrich), dark controls, and samples were analyzed using a Shimadzu Prominence high performance liquid chromatography (HPLC) system with a model SPD-20A/V UV-Vis absorbance detector set in dual wavelength mode at 371 and 435 nm. The HPLC column consisted of a Waters 8×100 mm Nova-Pak cartridge with 4 μm C18 packing placed in a Waters RCM radial compression cartridge holder (Waters Associates, Milford, MA). The mobile phase consisted of solvent A (Milli Q) and solvent B (acetonitrile). The elution program was isocratic at 30% B for 3 min, 30 to 55% B in 5 min, isocratic at 55% B for 2 min, 55 to 90% B in 6 min, isocratic at 90% B for 5 min, 90% B to 30% B in 1 min, followed by column equilibration to the initial mobile phase composition for 15 min. All samples were injected using a 1.25 mL injection loop. The flow rate was 1.5 mL min-1 and the column oven temperature was 40 ˚C. The sample analysis time was 37 min.
AQY method. AQY was calculated by dividing the moles of carbonyl compounds produced by the moles of photons absorbed by seawater. Refer to Zhu & Kieber (2018, 2019) for details regarding AQY determination, seawater absorptivity, and photon flux of the monochromatic irradiation.
Data processing was done in Excel
BCO-DMO processing notes:
- Conversion of coordinates: degrees decimal minutes to decimal degrees
- Added AQY_SetUp column to differentiate between two concatenated datafiles
- Renamed column headers to comply to database
| File |
|---|
aqy.csv (Comma Separated Values (.csv), 13.62 KB) MD5:af92e8a8877022f74e2a2af04d5bb356 Primary data file for dataset ID 781633 |
| Parameter | Description | Units |
| Sample_Date | Date of sample (UTC) in format YYYY-MM-DD | unitless |
| Station | Station identifier | unitless |
| Latitude | Latitude - South is negative | decimal degrees |
| Longitude | Longitude - West is negative | decimal degrees |
| Cast_ID_YYJSG | CTD Cast ID with format: YY=Year=16 (2016); J=Julian Day; S=Station; G=GMT | unitless |
| Depth_Sample_Collected | Depth below surface | meter (m) |
| Temperature | Temperature in Kelvin | Kelvin (K) |
| Wavelength | Wavelength | nanometer (nm) |
| Acetaldehyde | Apparent quantum yields (AQY) for the photochemical production of acetaldehyde in seawater - Carbonyl Photoproduction Efficiency | mole carbonyl photoproduced in seawater/mol photons absorbed by dissolved organic matter (mole/(mole quanta)) |
| Glyoxal | Apparent quantum yields (AQY) for the photochemical production of glyoxal in seawater - Carbonyl Photoproduction Efficiency | mole carbonyl photoproduced in seawater/mol photons absorbed by dissolved organic matter (mole/(mole quanta)) |
| Methylglyoxal | Apparent quantum yields (AQY) for the photochemical production of methylglyoxal in seawater - Carbonyl Photoproduction Efficiency | mole carbonyl photoproduced in seawater/mol photons absorbed by dissolved organic matter (mole/(mole quanta)) |
| AQY_SetUp | Wavelength or temperature dependent set-up | unitless |
| Dataset-specific Instrument Name | Shimadzu Prominence high performance liquid chromatography (HPLC) system |
| Generic Instrument Name | High-Performance Liquid Chromatograph |
| Dataset-specific Description | Shimadzu Prominence high performance liquid chromatography (HPLC) system with a model SPD-20A/V UV-Vis absorbance detector set in dual wavelength mode at 371 and 435 nm. |
| Generic Instrument Description | A High-performance liquid chromatograph (HPLC) is a type of liquid chromatography used to separate compounds that are dissolved in solution. HPLC instruments consist of a reservoir of the mobile phase, a pump, an injector, a separation column, and a detector. Compounds are separated by high pressure pumping of the sample mixture onto a column packed with microspheres coated with the stationary phase. The different components in the mixture pass through the column at different rates due to differences in their partitioning behavior between the mobile liquid phase and the stationary phase. |
EN589
| Website | |
| Platform | R/V Endeavor |
| Report | |
| Start Date | 2016-09-16 |
| End Date | 2016-10-15 |
| Description | The main purpose of this cruise was to study the organic matter put into the atmosphere as particles (also called aerosols) that are generated from bursting bubbles at the sea surface. To do this, the investigators deployed an aerosol generator to reproduce a model surface ocean using the ship's clean flow-through seawater system. The ship occupied four hydrographic stations: two biologically productive stations and two stations in the Sargasso Sea. To support the aerosol generator work, over fifty CTD casts were conducted to collect seawater and to characterize the physical, chemical, and biological properties of the water column.
Cruise description excerpted from EN589 post-cruise report: EN589_Post_Cruise_Report_10.20.16.pdf.
Related documents: EN589_Cruise_Plan.pdf |
Collaborative Research: Coupled Ocean-Atmosphere Recycling of Refractory Dissolved Organic Carbon in Seawater (Refractory DOC Recycling)
The oceans hold a massive quantity of organic carbon that is greater than all terrestrial organic carbon biomass combined. Nearly all marine organic carbon is dissolved and more than 95% is refractory, and cycled through the oceans several times before complete removal. Refractory dissolved organic carbon (RDOC) concentrations are uniform with depth in the water column and represent the "background" carbon present throughout the oceans. However, very little is known regarding RDOC production and removal processes. One potential removal pathway is through adsorption of RDOC onto surfaces of rising bubbles produced by breaking waves and ejection via bubble bursting into the atmosphere. Building on prior research, the investigators will evaluate the importance of ocean- atmosphere processing in recycling marine RDOC during a research cruise in the northwestern Atlantic Ocean. Results of the research will provide important insights regarding the coupled ocean-atmosphere loss of RDOC, thereby improving understanding of and ability to predict the role of RDOC in oceanic and atmospheric biogeochemistry, the global carbon cycle, and Earth's climate. The research will involve three early career faculty, and will provide training for undergraduate and graduate researchers.
Recent results based on a limited set of observations indicate that the organic matter (OM) associated with primary marine aerosol (PMA) produced by bursting bubbles from breaking waves at the sea surface is comprised partly to wholly of RDOC rather than OM of recent biological origin as has been widely assumed. The injection of RDOC into the atmosphere in association with PMA and its subsequent photochemical oxidation is a potentially important and hitherto unrecognized sink for RDOC in the oceans of sufficient magnitude to close the marine carbon budget and help resolve a long-standing conundrum regarding removal mechanisms for marine RDOC. This project will involve a shipboard investigation and modeling study to (1) quantify the relative contributions of marine refractory dissolved organic carbon (RDOC) to primary marine aerosol organic matter (PMA OM) produced from near-surface seawater in biologically productive and oligotrophic regions and from North Atlantic Deep Water, and to (2) determine the importance of atmospheric photochemical processing as a recycling pathway for RDOC. To test these hypotheses, a high-capacity aerosol generator will be deployed at four hydrographic stations in the NW Atlantic Ocean to characterize (1) the natural abundance of 14C in PMA and in surface and deep seawater; (2) the surface tension and physical properties of bubble plumes; (3) size-resolved production fluxes, chemical composition, organic carbon enrichments, spectral absorbance, and photochemical evolution of PMA; and (4) the carbon content, optical properties, and physical properties of seawater. The importance of RDOC recycling via PMA production and photochemical evolution will be interpreted with model calculations.
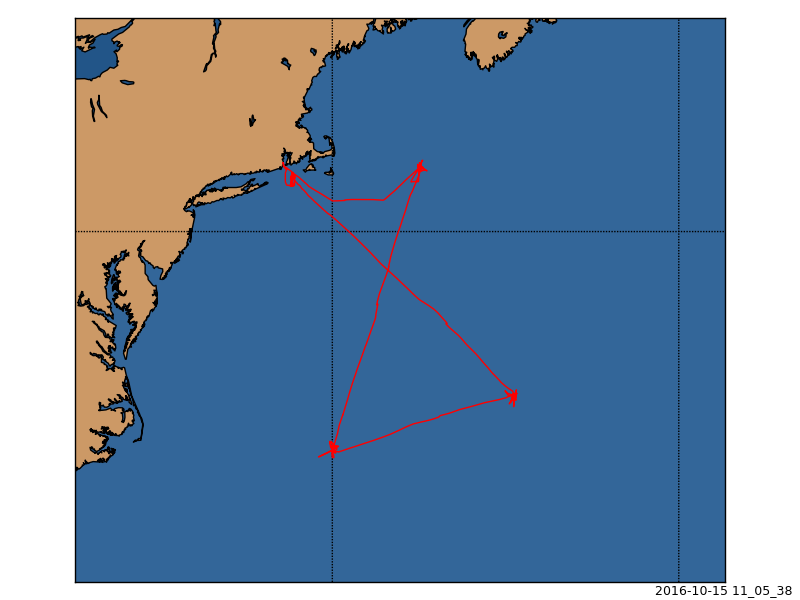
EN589 Cruise Track
United States Surface Ocean Lower Atmosphere Study (U.S. SOLAS)
The Surface Ocean Lower Atmosphere Study (SOLAS) program is designed to enable researchers from different disciplines to interact and investigate the multitude of processes and interactions between the coupled ocean and atmosphere.
Oceanographers and atmospheric scientists are working together to improve understanding of the fate, transport, and feedbacks of climate relevant compounds, and also weather and hazards that are affected by processes at the surface ocean.
Oceanographers and atmospheric scientists are working together to improve understanding of the fate, transport, and feedbacks of climate relevant compounds.
Physical, chemical, and biological research near the ocean-atmosphere interface must be performed in synergy to extend our current knowledge to adequately understand and forecast changes on short and long time frames and over local and global spatial scales.
The findings obtained from SOLAS are used to improve knowledge at process scale that will lead to better quantification of fluxes of climate relevant compounds such as CO2, sulfur and nitrogen compounds, hydrocarbons and halocarbons, as well as dust, energy and momentum. This activity facilitates a fundamental understanding to assist the societal needs for climate change, environmental health, weather prediction, and national security.
The US SOLAS program is a component of the International SOLAS program where collaborations are forged with investigators around the world to examine SOLAS issues ubiquitous to the world's oceans and atmosphere.
» International SOLAS Web site
Science Implementation Strategy Reports
US-SOLAS (4 MB PDF file)
Other SOLAS reports are available for download from the US SOLAS Web site
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) | |
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]